FYCOMPA received
its
initial approval as
adjunctive therapy
in
patients 12 years
of
age and older
with
uncontrolled
partial-onset
seizures based on 3
randomized,
double-blind,
placebo-controlled,
multicenter clinical
trials.1,**
During the 6-week titration period:1-4
•
>85% took 2 to 3
concomitant
AEDs.1
•
Patients were titrated
up on a
fixed
schedule, and
randomized to
one
of the maintenance doses
studied.2-4,†
During the 13-week
maintenance
period:1
•
Only patients
experiencing
intolerable
adverse reactions
were
given a dose
reduction.1,‡
*Median number of seizures per 28 days. Patients had more than 5 partial-onset seizures during the 6-week baseline period. 50% were taking at least 1 AED known to induce CYP3A4 (eg. carbamazepine, oxcarbazepine, or phenytoin).1
†Once-daily perampanel at 8 mg or 12 mg (Study 304 and Study 305) or at 2 mg, 4 mg, or 8 mg (Study 306).2-4
‡According to the investigators’ clinical judgment.4
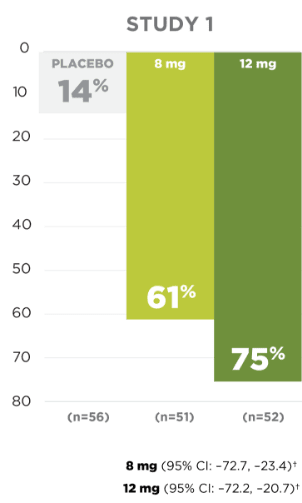
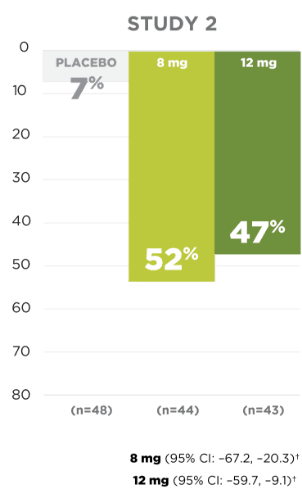
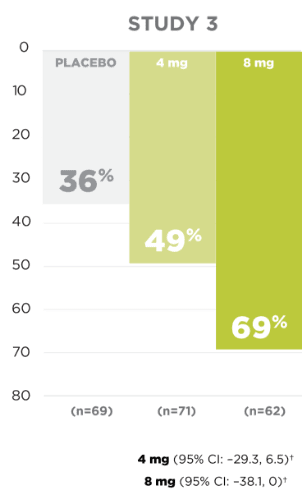
Seizure Frequency Reduction
A statistically significant decrease in all partial-onset seizures, with or without secondary generalizations, was observed at doses of 4 mg to 12 mg per day.1
PRIMARY ENDPOINT:
MEDIAN % REDUCTION IN SEIZURE FREQUENCY PER 28 DAYS





Efficacy with noninducer and inducer AEDs
MEDIAN % REDUCTION IN SEIZURE FREQUENCY
WITH NONINDUCER AND INDUCER AEDs1,5,*,†



*Patients from Latin American regions are excluded because of a significant treatment-by-region interaction due to high placebo response.1
† Concomitant enzyme-inducing AEDs (eg, carbamazepine, oxcarbazepine, or phenytoin) resulted in a substantial reduction in efficacy.1
Responder rate in the
pivotal clinical trials
for
partial-onset seizures
Responder rate was defined as the percentage of patients experiencing a 50% or greater reduction in partial-onset seizures.
SECONDARY ENDPOINT:
UP TO 54% OF PATIENTS
EXPERIENCED A ≥50% REDUCTION
IN
PARTIAL-ONSET
SEIZURE FREQUENCY1,5




*Patients from Latin American regions are excluded because of a significant treatment-by-region interaction due to high placebo response.1
†Concomitant enzyme-inducing AEDs (eg, carbamazepine, oxcarbazepine, or phenytoin) resulted in a substantial reduction in efficacy.1
Reduction in secondarily generalized convulsive seizure frequency
SUBGROUP ANALYSIS:
MEDIAN % REDUCTION IN SEIZURE FREQUENCY PER 28 DAYS*


LIMITATIONS
Prespecified exploratory endpoint not adjusted for multiplicity and not adequately powered to show statistical significance.
*Analysis included patients who experienced this seizure type during the baseline period.†Confidence intervals reflect lower and upper limit of the median difference to placebo.
Secondarily
generalized
convulsive
seizures
freedom rates
Seizure freedom rates in
secondarily generalized
convulsive seizures5
UP TO
36%
OF PATIENTS
(1 in 3)
with
secondarily
generalized
seizures were
Convulsive
Seizure
Free1,*,†
during the 13-week
maintenance
phase
LIMITATIONS
Secondarily generalized
seizure freedom
was
a post-hoc analysis not
adjusted for
multiplicity
and not
adequately powered to
show
statistical significance.
These
analyses
for seizure freedom are
descriptive.
*Analysis
included patients who
experienced this seizure
type during the baseline
period.
†Seizure freedom during
maintenance period in
patients who completed the
Phase 3 study maintenance
phase.
•
The following ARs were
dose-related
and
occured mostly during the
titration
phase:
dizziness and disturbance in
gait
or
coordination (including
ataxia, gait disturbance,
balance disorder, and
abnormal
coordination), somnolence
and fatigue-related events
(including fatigue,
asthenia, and lethargy).1
•
For almost every adverse
reaction, rates were higher
on 12 mg and more often
led
to dose reduction or
discontinuation.1
Discontinuation rates rose with FYCOMPA dosage
PATIENTS WHO DISCONTINUED DUE
TO AN
ADVERSE REACTION, BY
DOSE1


The adverse reactions most
commonly leading to
discontinuation (≥1% in the 8 mg
or 12 mg/day
FYCOMPA group and greater than
placebo) were dizziness,
somnolence, vertigo, aggression,
anger,
ataxia, blurred vision,
irritability, and
dysarthria.1
Dizziness and disturbance in
gait or coordination led to
discontinuation in 3% of
FYCOMPA-treated
patients vs 1% of
placebo-treated patients.
Somnolence or fatigue-related
events led to discontinuation
in 2% of FYCOMPA-treated
patients and 0.5% of
placebo-treated patients.
Elderly patients had an
increased risk of these adverse
reactions compared to younger
adults and pediatric
patients.1
REFERENCES: 1.
FYCOMPA US
Prescribing
Information. Coral Gables, FL:
Catalyst Pharmaceuticals, Inc.
2.
French JA, Krauss GL,
Biton
V, et al. Adjunctive perampanel
for
refractory
partial-onset seizures:
randomized
phase III study 304.
Neurology . 2012 Aug 7;79(6):589-96.
3.
French JA, Krauss GL, Steinhoff
BJ,
Squillacote
D, Yang H, Kumar D, Laurenza
A.
Evaluation of adjunctive
perampanel in
patients
with refractory partial-onset
seizures:
results of randomized global
phase
III study 305.
Epilepsia . 2013
Jan;54(1):117-25.
4.
Krauss GL, Serratosa
JM,
Villanueva V, Endziniene M, Hong
Z,
French
J, Yang H, Squillacote D,
Edwards
HB,
Zhu J, Laurenza A. Randomized
phase
III
study 306: adjunctive perampanel
for
refractory
partial-onset seizures.
Neurology . 2012 May 1;78(18):1408-15.
5.
Data on file. Catalyst
Pharmaceuticals Inc., Coral
Gables, FL.
IMPORTANT SAFETY
INFORMATION AND
INDICATION
IMPORTANT SAFETY
INFORMATION
AND INDICATION
WARNING: SERIOUS PSYCHIATRIC AND BEHAVIORAL REACTIONS
•
Serious or
life-threatening
psychiatric and
behavioral
adverse
reactions
including
aggression,
hostility,
irritability,
anger, and
homicidal
ideation
and
threats have
been reported in
patients taking
FYCOMPA
• These reactions occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression
• Advise patients and caregivers to contact a healthcare provider immediately if any of these reactions or changes in mood, behavior, or personality that are not typical for the patient are observed while taking FYCOMPA or after discontinuing FYCOMPA
• Closely monitor patients particularly during the titration period and at higher doses
• FYCOMPA should be reduced if these symptoms occur and should be discontinued immediately if symptoms are severe or are worsening
SERIOUS PSYCHIATRIC AND BEHAVIORAL REACTIONS
In the partial-onset seizures clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. These effects in FYCOMPA-treated patients led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients. Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol. Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least one month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic (PGTC) seizure clinical trial.
SUICIDAL BEHAVIOR AND IDEATION
Antiepileptic drugs (AEDs), including FYCOMPA, increase the risk of suicidal thoughts or behavior in patients. Anyone considering prescribing FYCOMPA or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Patients, their caregivers, and families should be informed of the risk and advised to monitor and immediately report the emergence or worsening of depression, suicidal thoughts or behavior, thoughts about self-harm and/or any unusual changes in mood or behavior. Should suicidal thoughts and behavior emerge during treatment, consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
DIZZINESS AND GAIT DISTURBANCE
FYCOMPA caused dose-related increases in events related to dizziness and disturbance in gait or coordination. Dizziness and vertigo were reported in 35% and 47% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 10% of placebo-treated patients. Gait disturbance related events were reported in 12% and 16% of patients in the partial-onset seizure clinical trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 2% of placebo-treated patients. These adverse reactions occurred mostly during the titration phase. These adverse reactions were also observed in the PGTC seizure clinical trial.
SOMNOLENCE AND FATIGUE
FYCOMPA caused dose-dependent increases in somnolence and fatigue-related events. Somnolence was reported in 16% and 18% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 7% of placebo-treated patients. Fatigue-related events were reported in 12% and 15% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 5% of placebo-treated patients. These adverse reactions occurred mostly during the titration phase. These adverse reactions were also observed in the PGTC seizure clinical trial. Patients should be advised against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of FYCOMPA is known. Patients should be carefully observed for signs of central nervous system (CNS) depression when FYCOMPA is used with other drugs with sedative properties because of potential additive effects.
FALLS
Falls were reported in 5% and 10% of patients in the partial-onset seizure clinical trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 3% of placebo-treated patients.
DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS (DRESS)
DRESS, also known as multiorgan hypersensitivity, has been reported in patients taking AEDs, including FYCOMPA. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement. If signs or symptoms are present, immediately evaluate the patient and discontinue FYCOMPA if an alternative etiology for signs or symptoms cannot be established.
WITHDRAWAL OF AEDs
A gradual withdrawal is generally recommended with AEDs to minimize the potential of increased seizure frequency, but if withdrawal is a response to adverse events, prompt withdrawal can be considered.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions in patients aged 12 years and older receiving FYCOMPA (≥5% and ≥1% higher than placebo) include dizziness, somnolence, fatigue, irritability, falls, nausea, weight gain, vertigo, ataxia, headache, vomiting, contusion, abdominal pain, and anxiety. Adverse reactions in patients 4 to <12 years were generally similar to patients aged 12 years and older.
DRUG INTERACTIONS
FYCOMPA may decrease the efficacy of contraceptives containing levonorgestrel. Plasma levels of perampanel were decreased when administered with known moderate and strong CYP3A4 inducers, including, carbamazepine, phenytoin, or oxcarbazepine. Multiple dosing of FYCOMPA 12 mg per day enhanced the effects of alcohol on vigilance and alertness, and increased levels of anger, confusion, and depression. These effects may also be seen when FYCOMPA is used in combination with other CNS depressants.
PREGNANCY AND LACTATION
Physicians are advised to recommend that pregnant patients taking FYCOMPA enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Caution should be exercised when FYCOMPA is administered to pregnant or nursing women as there are no adequate data on the developmental risk associated with use in pregnant women, and no data on the presence of perampanel in human milk, the effects on the breastfed child, or the effects of the drug on milk production.
HEPATIC AND RENAL IMPAIRMENT
Use in patients with severe hepatic or severe renal impairment is not recommended. Dosage adjustments are recommended in patients with mild or moderate hepatic impairment. Use with caution in patients with moderate renal impairment.
DRUG ABUSE AND DEPENDENCE
FYCOMPA is a Schedule III controlled substance and has the potential to be abused and lead to drug dependence and withdrawal symptoms including anxiety, nervousness, irritability, fatigue, asthenia, mood swings, and insomnia.
INDICATION
FYCOMPA® (perampanel) is indicated in patients with epilepsy aged 4 years and older for partial-onset seizures (POS) with or without secondarily generalized seizures and adjunctive therapy for patients aged 12 years and older for primary generalized tonic-clonic (PGTC) seizures.
View the Prescribing Information





